Why do we need Class 6.2 Infectious Substance Packaging?
Infectious substance packaging is intended to enable the safe transportation of infectious substances. It is designed to prevent an exposure occurring in the event where the infectious substances’ primary receptacle is compromised, resulting in a leakage of the infectious substance.
The protective packaging therefore must contain the leakage preventing it from being released outside of the packaging and coming in contact with humans or animals. You can’t just use any old packaging, the packaging you choose must be packaging that has passed specific performance testing, we’ll come on to that in a bit…
What is an Infectious Substance?
Infectious Substances are defined as; substances which are known or are reasonably expected to contain pathogens. Pathogens are defined as micro-organisms (including; bacteria, viruses, rickettsiae, parasites, fungi) and other agents such as prions, which can cause diseases in humans or animals.
What are the UN numbers for Infectious Substances?
All Class 6 – Infectious Substances (Division 6.2), are or will be assigned to one of five UN Numbers;
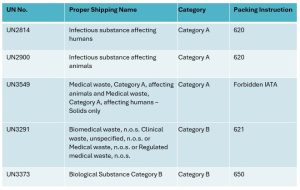
UN2814, Infectious substance, affecting humans
UN2900, Infectious substance affecting animals
UN3549, Medical waste, Category A, affecting animals only, solid.
UN3549, Medical waste, Category A, affecting humans, solid.
UN3291, Clinical waste unspecified n.o.s or (Bio) medical waste n.o.s or Regulated medical waste n.o.s
UN3373, Biological Substance Category B
How do the categories work?
Infectious substances that come under Class 6 Infectious Substances (Division 6.2) are divided into 3 categories.
Category A; An infectious substance that if an exposure occurs when transported is capable of causing permanent disability, life threatening or fatal disease in otherwise healthy humans.
Category B; An infectious substance which does not meet the criteria for inclusion in Category A.
Exceptions; There are a number of exceptions listed in the regulations, for example; substances that are unlikely to cause disease in humans or animals, patient specimens where there is a minimum likelihood that pathogens are present, or medical equipment potentially contaminated with or containing infectious substances. You must refer to the relevant regulations to identify whether your substance falls under exceptions as some exceptions do require specific packaging when transporting.
Packing instructions
When transporting any of the 5 UN numbers listed above, you must adhere to the relevant packing instructions for the mode of transport(s) used, in this example we use IATA packing instructions.
UN2814 = P620
UN2900 = P620
UN3549 = Forbidden
UN3291 = P621
UN3373 = P650
Category A and B IATA Packing Instructions Table
How to package your Infectious Substance for safe transportation
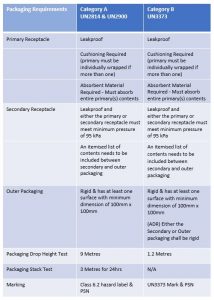
Infectious Substances that fall in both Category A and Category B (excluding UN3291) require triple packaging; this consists of:
- Leakproof primary receptacle(s)
- Leakproof secondary packaging
- Absorbent material
- Cushioning (if more than 1 primary receptacle, they must be individually wrapped)
- Rigid outer *UN approved packaging (*UN Approved required for Category A only)
All outer packaging that is designed to carry Category A Infectious Substances must be UN approved, which means, way back in the design phase of the packaging, the packaging manufacturer must adhere to strict guidelines and ensure the packaging passes a number of tests to prove it can contain the infectious substance should a leak occur. To gain UN approval, the manufacturer’s designed package is sent to and tested by an independent testing company, if the packaging successfully passes the tests, it is then sent for verification to the relevant state approval authority for approval.
Once the relevant state of approval authority verifies the packages performance and is happy the packaging adheres to all requirements, a UN mark is assigned to the packaging and a certification is issued. The packaging design can then go into production for commercial use. An important note but one often overlooked is that when using UN approved packaging, it must be assembled and the infectious substance packed in the exact same way that the packaging was tested for. UN approved packaging used incorrectly will invalidate the performance certification. To ensure you package the infectious substance correctly, make sure your packaging supplier provides assembly instructions with the packaging you choose for your infectious substances.
Unlike packaging for Category A Infectious Substances, packaging for Category B does not have to be UN approved, however, it does need to have demonstrated that it can pass certain performance tests.
Packaging and test requirements for Category A and Category B Infectious Substances.
In summary
In order to ship compliantly, the shipper must use the correct packaging for Category A and Category B Infectious Substances and ensure it is assembled as per the packaging manufacturer’s instructions.
So, you know you are purchasing compliant packaging, you may want to ask your packaging manufacturer to provide you proof of certification that their packaging meets the require testing standards for shipping infectious substances.
It is worth noting that as Category A packaging has been approved using more advanced testing requirements than Category B packaging, Category B Specimens can be shipped in Category A packaging should you chose to, however Category A specimens are not permitted to be packaged in Category B packaging.
For Category A and B packaging options click here
Information correct at time of publishing May 2020
*Last updated November 2024
 US
US