If you are involved in the handling of infectious substances at any stage of the shipping process, then you will need to be aware of the restrictions that apply to shipping class 6.2 Infectious substances within the UN classification regulations.
Class 6.2 refers to infectious substances. This is made up of substances that contain, or are reasonably expected to contain, pathogens that can cause disease in humans or animals. For example:
- Bacteria
- Bacillus anthracis, streptococcus, E. coli
- Fungi
- Candida, cryptococcus neoformans, mucormycetes
- Parasites
- Ancylostomiasis, cysticercosis, filariasis
- Viruses
- Ebola virus, monkeypox virus, lumpy skin disease virus
Here at Air Sea USA, we can supply you and your business with packaging solutions that are suitable for transporting infectious substances. When transporting Class 6.2 Infectious Substances it is imperative that shippers use the correct packaging as specified in the relevant dangerous goods regulations for your chosen method(s) of transport.
Who might this information apply to?
A high percentage of class 6.2 packages that are shipped are medical samples. This is in part due to the fact that any human or animal infectious substances which are reasonably expected to contain, pathogens, as a minimum are classed as Category B class 6.2 infectious substances.
Shipments of Class 6.2 samples are often required to be transported to or from hospitals, prisons or veterinary clinics, as class 6.2 includes substances such as biological products, cultures, patient specimens and medical or clinical waste, those of which can cause disease in humans or animals.
Information in this article will be relevant to any business that handles infectious substances being shipped, regardless of whether they are the consignor (sender), carrier, or consignee (receiver).
However, as the consigner is responsible for ensuring that the infectious substances shipment is packaged and labelled correctly, they will benefit most from the shipping guidance given.
For example, we have a lot of customers who have a requirement to send class 6.2 substances relating to veterinary samples, and some who are required to send biological samples (category B) via the US postal service. We hope that this information will help make their job safer and easier.
We encourage any parties involved in packaging infectious substances to receive further packaging guidance from ourselves if they are unclear on any of the official regulations.
How to package class 6.2 samples
The UN Model Regulations have produced four sets of packing instructions that stipulate how infectious substances must be packaged.
These instructions are as follows:
- P620 – Category A (UN2814 and UN2900)
- P650 – Category B (UN3373)
- P621 – Category B medical or clinical waste (UN3291)
- P622 – Category A medical waste affecting humans or animals (UN3549)
Packing instructions P620 and P650 build off a basic triple packaging system that is comprised of three layers: primary receptacle, secondary packaging, and outer packaging. An absorbent material should also be included that can absorb all of the substance being shipped in the event of a break or leak.
Packing instructions P621 does not follow the triple packaging system and other forms of packaging are allowed, for example a rigid Outer packaging – Drums, Jerricans & Boxes are acceptable provided that the packaging meets UN packing group II performance standards.
Packing Instruction P622 requires triple packaging consisting of inner, intermediary and outer packagings. The outer packaging must conform to UN packing group I performance level for solids.
It is possible to post UN3373 substances using the postal service. UN3373 US postal service compliant packaging is available on our website.
Packaging that is suitable for transporting category A infectious substances will also be suitable for transporting category B infectious substances – but not vice versa.
Primary receptacle
The infectious substances should be stored within a leakproof (for liquids) or a siftproof (for solids) primary receptacle. It should be made of glass, plastic, or metal, and labelled in accordance to the dangers of the goods within.
If transporting a liquid, an absorbent material that can absorb 100% of the liquid should be stored alongside the primary receptacle.
Secondary packaging
The secondary packaging should also be watertight and suitably leakproof or siftproof. This packaging should fully enclose the primary receptacle and absorbent material.
For UN3373 substances, either the secondary packing or the outer packaging must be rigid. Whereas for UN2814 and UN2900 substances, the outer packing must be rigid.
In some cases, it is possible to transport more than one primary receptacle within the secondary packaging (i.e., if all substances are the same class). If so, each primary receptacle must be stored in a way that prevents contact or breakage between them – a cushioning material may be used.
Outer packaging
Outer packaging is the final layer of triple packaging and it should fully enclose the secondary packaging and primary receptacle.
Because the outer packaging is the last barrier for protecting the goods within, it must be of an appropriate strength and capable of withstanding any damages that may occur during transit.
There should be an itemised list of the contents being transported located either between the secondary packaging and outer packaging or taped onto the exterior of the secondary packaging.
It is required for at least one surface of the outer packaging to have a minimum dimension of 100mm × 100mm.
Class 6.2 shipping exemptions
There are a number of circumstances where a shipper may be exempt from following the class 6.2 shipping regulations for infectious substances.
For example, when shipping human and animal medical samples for testing unrelated to infectious diseases (where there is a low probability that any pathogens will be present), consignors can mark the outer packaging as ‘exempt human specimen’ or ‘exempt animal specimen’ as appropriate and avoid other infectious substance regulations.
UN3291 and UN3549 medical waste are not permitted for exemption and should continue to be classified as infectious substances.
For more information on exemptions, please contact our team at Air Sea USA and we can offer you advice.
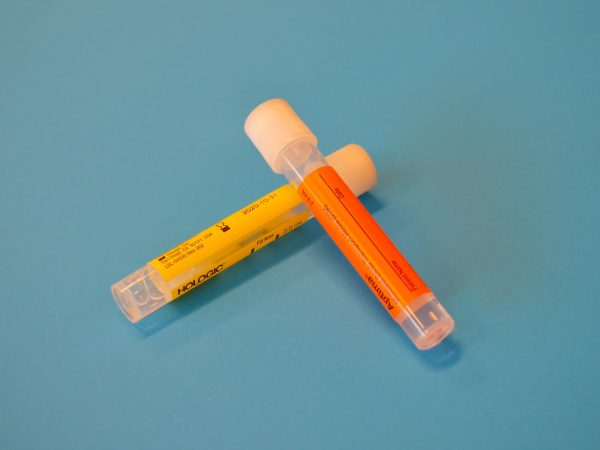
Class 6.2 shipping products for infectious substances
At Air Sea USA, we stock complete triple packaging systems and individual components for both category A (UN2814 and UN2900) and category B (UN3373) infectious substances.
If you cannot see any products that meet your needs, then please contact us for a custom made infectious substance packaging quote and we would be happy to help.
Air Sea USA infectious substance packaging
We hope that we have helped you determine what infectious substance packaging you need. If you require any further advice from us regarding sending infectious substances by post, or any other UN approved packaging subject, contact us and we will get back in touch with our advice.
 US
US